04Clinical Trials
A placebo-controlled, randomized, double-blind comparative study.
| Inclusion criteria | Cats with CKD that meet all the following criteria: Cats with serum creatinine≥1.6 mg/dL, urine specific gravity< .035, T4 0.9–3.8 µg/dL |
|---|---|
| Animals used | Client-owned cats with IRIS stage 2 and 3 CKD. Efficacy evaluation: n = 63 (RAPROS® treatment group n = 31, Placebo treatment group n = 32) Safety evaluation: n = 74 (RAPROS® treatment group: n = 36, Placebo treatment group: n = 38) |
| Treatment | Cats were orally administered 55µg beraprost or placebo twice daily |
| Treatment period | 180 days |
| Safety criteria | General condition, blood test and occurrence of adverse events |
| Efficacy criteria |
Serum creatinine, BUN, body weight, clinical score* (appetite, activeness, dehydration), QOL evaluation by owner and overall improvement evaluation by veterinarian, etc. * Clinical Score
|
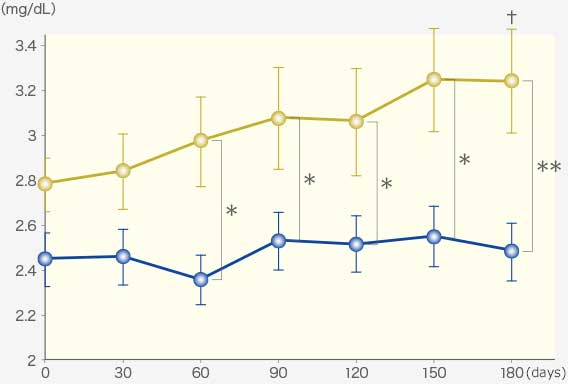
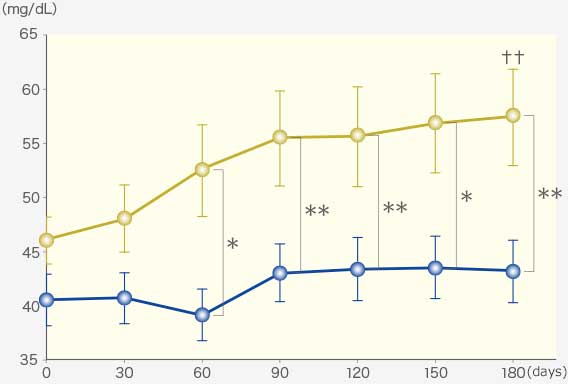
RAPROS® inhibited increase in serum creatinine and BUN1.
Serum creatinine
BUN
Data submitted to Ministry of Agriculture,
Forestry and Fisheries of Japan
- RAPROS®treatment group
- Placebo treatment group
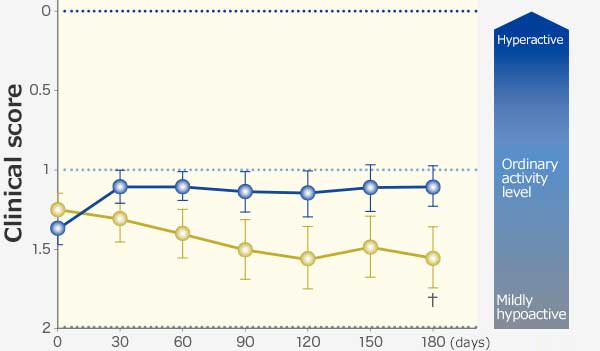
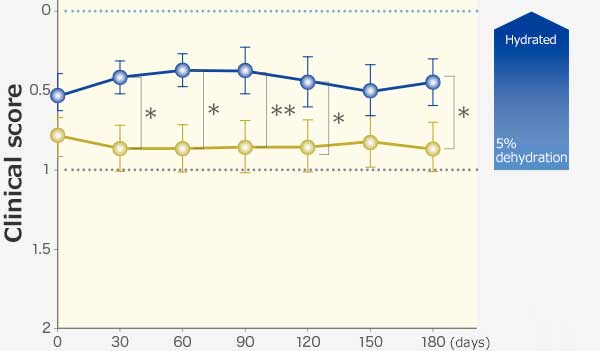
Comparison within groups (pre vs post): Repeated ANOVA (♰: P <0.01, ♰♰: P <0.001)
Comparison between groups (RAPROS® treatment group vs Placebo treatment group): Factorial ANOVA (*: P <0.05, **P <0.01)
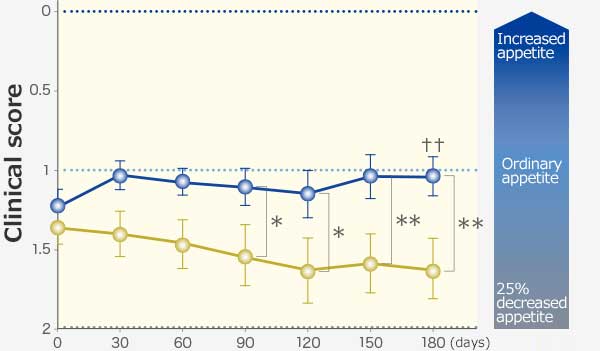
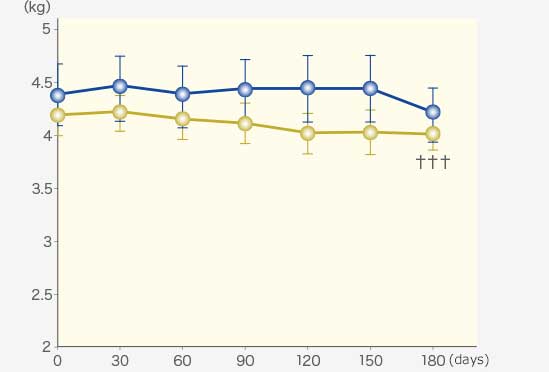
RAPROS® improved anorexia and loss of body weight. It also improved hypoactivity1.
Changes in body weight and clinical scores
Data submitted to Ministry of Agriculture,
Forestry and Fisheries of Japan
- RAPROS®treatment group
- Placebo treatment group
Comparison within groups (pre vs post): Repeated ANOVA (♰: P <0.01, ♰♰: P <0.001)
Comparison between groups (RAPROS® treatment group vs Placebo treatment group): Factorial ANOVA (*: P <0.05, **P <0.01)
Appetite
Body Weight
Physical Activity
Dehydration
Safety assessment of RAPROS®
All adverse events were reviewed regardless of causal relationship with RAPROS® or placebo1.
Main adverse events
| Causal relationship with treatment drug | Treatment group | Adverse events (severity) | Number of cases |
|---|---|---|---|
| Known | Placebo group | Vomiting (minor) | 1 case |
| Unknown | RAPROS®group | Decrease in appetite and body weight (mild) | 1 case |
| Placebo group | Stroke (mild) | 1 case | |
| Vomiting blood (mild) | 1 case | ||
| Increase in hepatic enzyme level (moderate) | 1 case |
- Pilot study on target animal safety2
- Healthy cats were given up to 3 times the clinical dosage of RAPROS® for 182 days.
Clinical signs, body weight, food consumption, water consumption, urinalysis, hematology, blood biochemistry, blood coagulation, ophthalmology, electrocardiography, necropsy, and histology results were recorded. None of these parameters showed any changes attributable to RAPROS® administration. Data submitted to Ministry of Agriculture, Forestry and Fisheries of Japan
References
- 1) Takenaka, M., Iio, A., Sato, R., Sakamoto, T., Kurumatani, H. and (2018), A Double-blind, Placebo-controlled, Multicenter, Prospective, Randomized Study of Beraprost Sodium Treatment for Cats with Chronic Kidney Disease. J Vet Intern Med, 32: 236-248. https://doi.org/10.1111/jvim.14839
- 2) Pre-publishing research data of Toray Industries, Inc.

